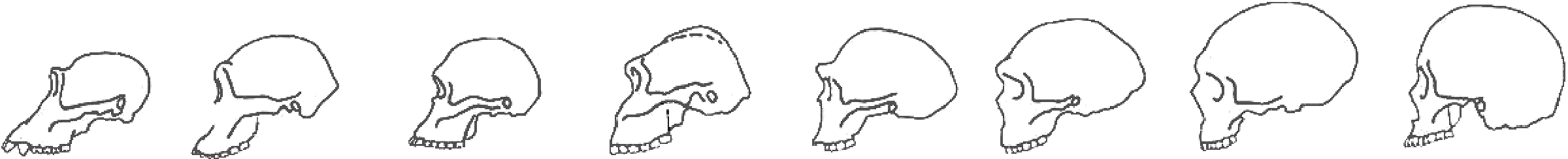Short Story of Human Evolution

Cassandra M. Turcotte
Center for the Advanced Study of Human Evolution
Center for the Advanced Study
of Human Evolution
George Washington University
How humans became 'human': Cassandra Turcotte of the Center for the Advanced Study of Human Evolution considers one of our most important questions. Human origins is being investigated and understood through evolutionary theory, which sees humans placed with the other great apes on the Tree of Life. We must now look at 3 fundamental questions: how and why the human and ape lineages diverged and, thirdly, what morphological, genetic and behavioral changes occurred during human evolution to bring about modern 'humanness'.
The question of how humans came to be has been one of the longest standing quandaries known to man, first posed far before the advent of modern science. According to popular myth from the Ancient Greek world, for example, humans arose from the dirt through the intervention of the Titan Prometheus. Since then, however, researchers have come to understand human origins in a much more detailed, evidence-based way. Thanks to the last few centuries' advances in evolutionary theory, humans are now placed with the other great apes on the Tree of Life. Specifically, chimpanzees share the closest genetic relationship among all of the apes with humans and this suggests that the question of human origins really consists of three sub-questions. That is, researchers must answer how and why the human and chimpanzee lineages diverged and, once they did, what morphological, genetic and behavioral changes occurred during human evolution to bring about modern humanness.
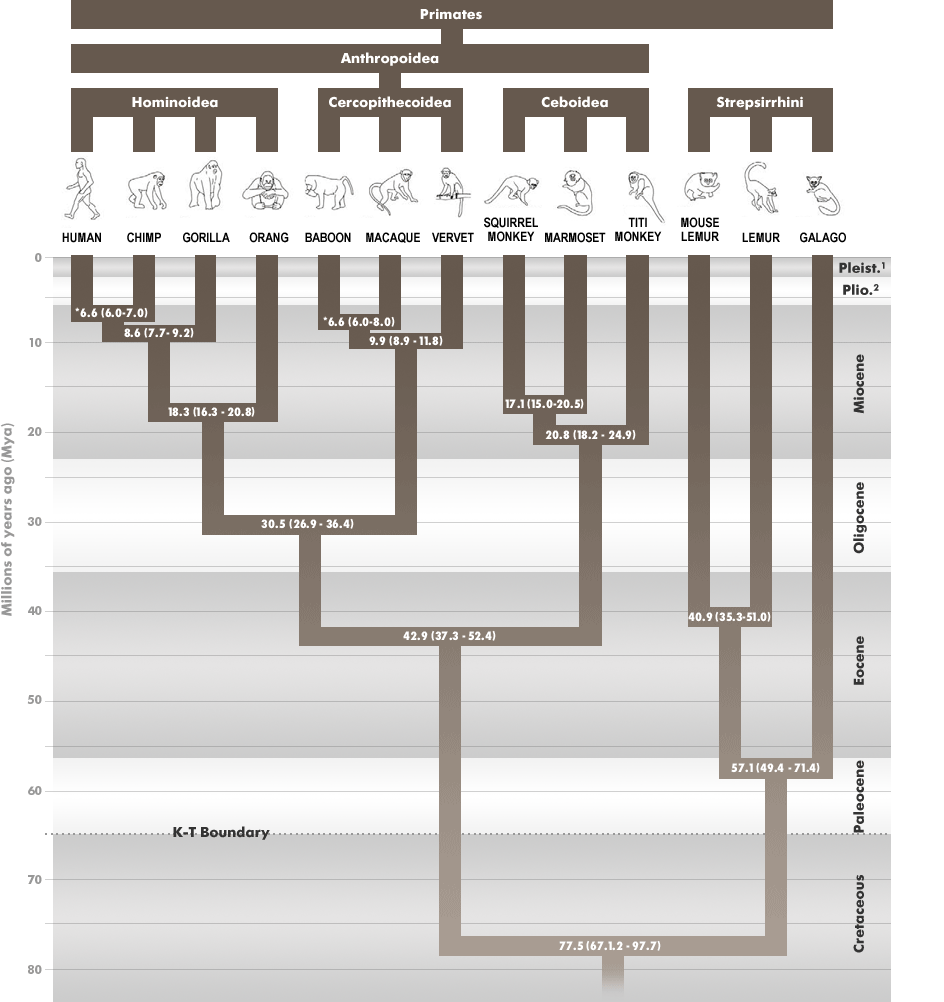
Figure 1: The reconstructed phylogeny of the Order Primates showing high and low divergence dates for each node (from Steiper and Young, 2006)
Such a broad question requires several lines of evidence. The problem relating to the human-chimpanzee divergence, for instance, has been addressed both through genetic and morphological analyses. With genetic material, researchers can evaluate commonalities in the nuclear and mitochondrial genome as a measure of interrelatedness and to date divergence times (Steiper and Young, 2006; Wood, 2005). In figure 1, a reconstructed phylogeny of the Order Primates, including high and low estimates for dates of divergence, shows the common grouping of orangutans; gorillas; chimpanzees and humans into the Superfamily Hominoidea. Furthermore, it reflects the relationship of the human and chimpanzee lineage and places their split around 6-7 million years ago (Steiper and Young, 2006). Details of this divergence, including what the common ancestor of humans and chimpanzees looked like, depend on additional morphological evidence.

Figure 2: Knuckle walking ancestor
Traditionally, this last common ancestor (LCA) was reconstructed in particular ways to emphasize its primitiveness and similarity to the chimpanzee, even though the chimp is as temporally distant from the LCA as humans. One of the primary issues regarding the reconstruction involves locomotor mode. Typically, researchers view this ancestor as engaging in knuckle-walking, the locomotor behavior shared by chimps and gorillas (Lovejoy et al., 2009; Lovejoy et al., 2009b). Knuckle-walking traits consist of elongated palms, bent fingers, and stiff wrists to stabilize the hand when it bears weight. Therefore, it's always been a source of confusion that modern humans as well as fossil australopiths do not show those remnants of knuckle-walking ancestry, which researchers would expect if the human lineage had indeed descended from a knuckle-walking ancestor (Lovejoy et al., 2009b; Harcourt-Smith, 2010). The truth is that reconstructions of the LCA are hypothetical and speculative.
Part of the reason for the speculative nature of these reconstructions lies in the scarcity of fossil evidence from the time period in question. Currently only fragments of highly contested fossils are known to represent the human lineage from about 4 Ma and older. No fossil panins have been recovered for dates earlier than 700 kyr (Wood, 2005). This could be due either to selective preservation during fossilization of early humans occupying different environmental niches or a researcher bias for identifying fossils as part of the human rather than the chimpanzee lineage (Wood, 2005). Despite over a century of fossil hunting, however, the early years of the human lineage remain a largely unknown period.
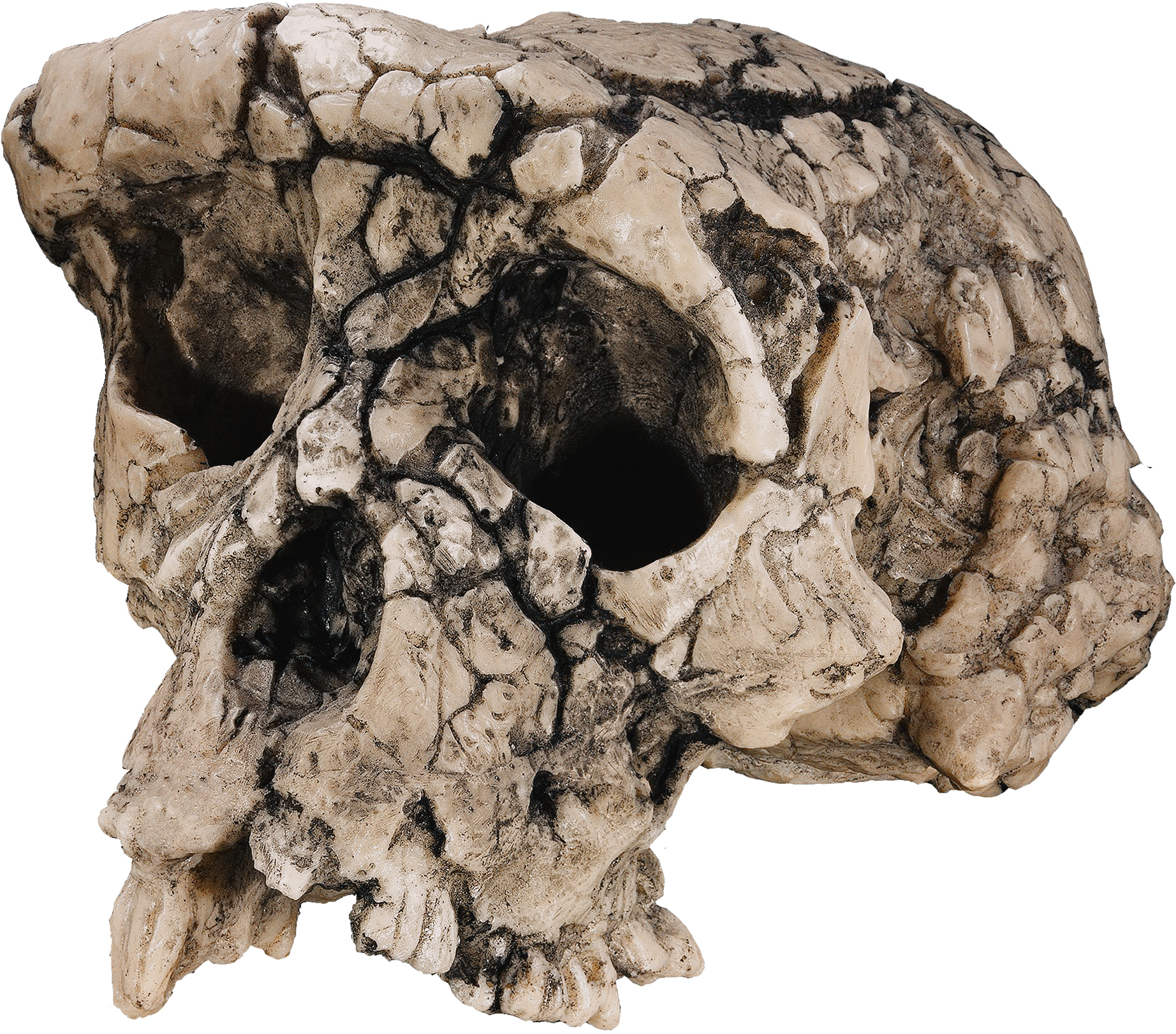
Figure 3: Sahelanthropus tchadensis
The current contenders for the coveted title of earliest human ancestor number just three. The oldest of the group at 7 Ma, the species
Sahelanthropus tchadensis, hails from Toros-Menalla in Chad and consists of just one distorted cranium plus two mandibles (Brunet et al., 2002). Features of this fossil include a chimp-sized brain, gracile brow ridge, robust lower jaw and human-like tooth wear in what Brunet et al. (2002) describe as a mosaic of primitive and derived characters that place the fossil at the base of the human branch. Those who are skeptical of this interpretation, nevertheless, point to the fossil's overall robusticity and geography to claim that it may represent an hitherto unknown fossil ape (Wood, 2005).
The picture doesn't get much clearer with the introduction of 6 Ma
Orrorin tugenensis, that includes a set of molars and a femoral fragment from the Tugen Hills of northern Kenya (Senut et al., 2001). Senut et al. (2001) claimed that these fragments belong on the human lineage because of the thick enamel covering the molar and premolar teeth and the internal morphology of the femoral neck. Supposedly, locomotor mode leaves signature patterns of differential distribution of cortical bone in the femoral neck, with habitual bipeds exhibiting thickening of such bone at the top and bottom of the neck. Problematically, it would be rash to assume that thick tooth enamel is exclusive to humans and, more importantly, CT scan technology hasn't actually managed to capture a distinct enough image of the femoral neck to comment on cortical bone morphology (Wood, 2005). The incompleteness of these fossils is a major factor in their resistance to categorization but it is not the only reason.
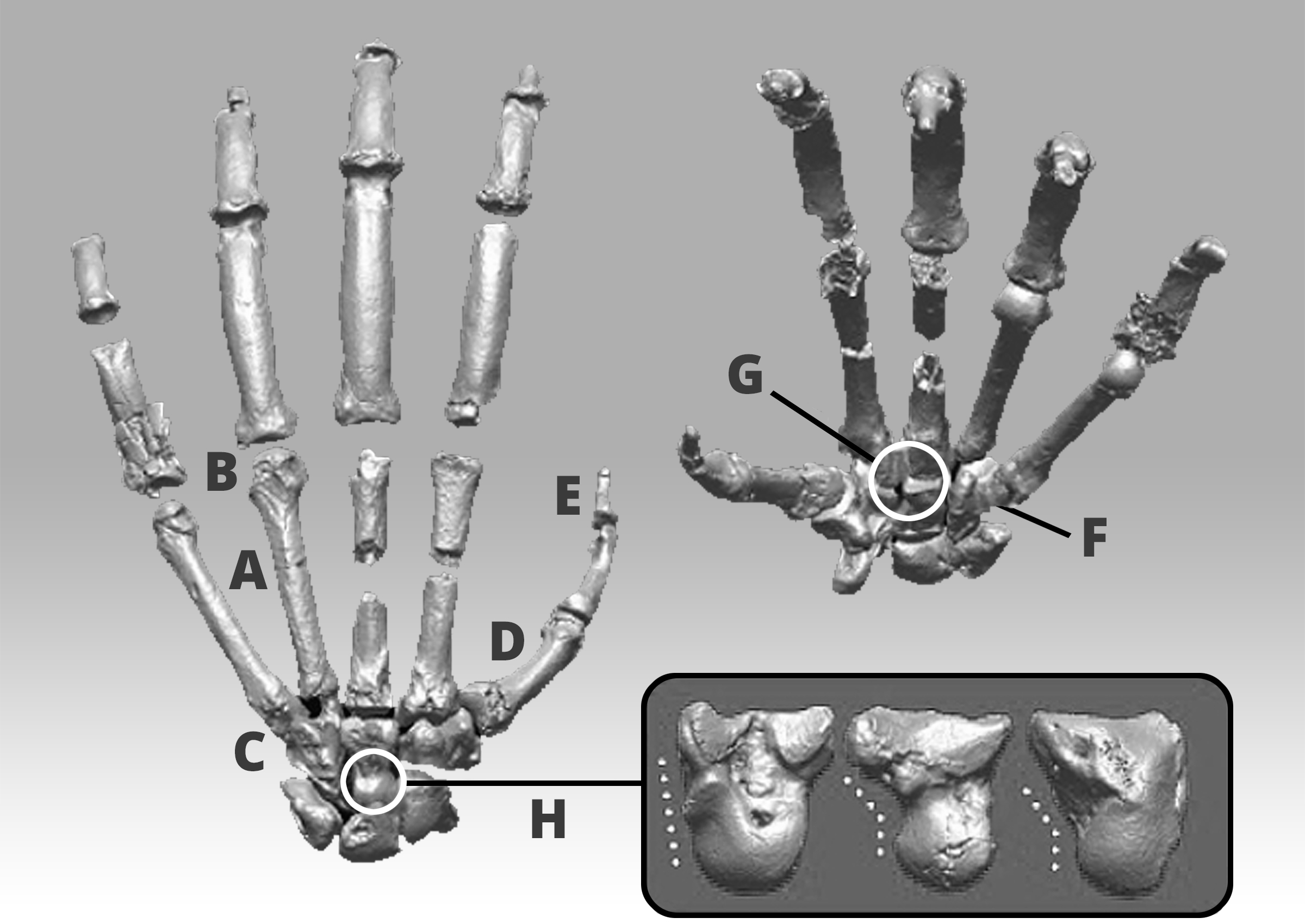
Figure 4: Prone and supine views of the left hand of
Ardipithecus ramidus illustrating primitive features that don't appear in more specialized apes such as chimps.
A) Short metacarpals;
B) Absence of knuckle-walking grooves;
C) Extended joint surface on the front and back of the fifth digit joint surface;
D) Robust thumb;
E Insertion for long flexor tendon;
F) Hamate, allowing palm to flex;
G) Simple wrist joints;
H) Capitate head promoting palm flexion. Inset: lateral views of the Pan,
Ardipithecus ramidus, and human capitates left to right
The collection of fossils attributed to the third fossil species, Ardipithecus ramidus, for example, is more complete than that of either
Sahelanthropus or
Orrorin, but its status is even more vigorously disputed. The controversy surrounding
Ardipithecus ramidus is mostly to do with its putative bipedal locomotor capabilities, one of the features often used to distinguish early humans from early chimpanzees.
Ardipithecus ramidus has an interesting mosaic of features, which its authors interpret as evidence of palmigrade quadrupedality grading into facultative bipedalism. According to Lovejoy et al. (2009b) palmigrade quadrupedality only took place in the trees; on the ground, they claim, Ardipithecus engaged in facultative bipedalism. In support of palmigrady, Lovejoy et al. (2009b) cite Ardipithecus' metacarpal head, among other traits [Fig. 2], as evidence. The dorsum of the metacarpal heads exhibits symmetrical grooves where the collateral ligaments would have attached in life and increased dorsiflexion sufficient for palmigrady (Lovejoy et al., 2009; 2009b). In addition to exhibiting the morphological characters associated with palmigrade quadrupedality,
Ardipithecus ramidus is suggested to also have bipedal indicators, such as a more forward facing foramen magnum; stiff, fibrous feet; and a more human-like hip (Lovejoy et al., 2009b; 2009c). More definitive evidence of bipedal walking, however, doesn't show up in the fossil record until the emergence of
Australopithecus afarensis.
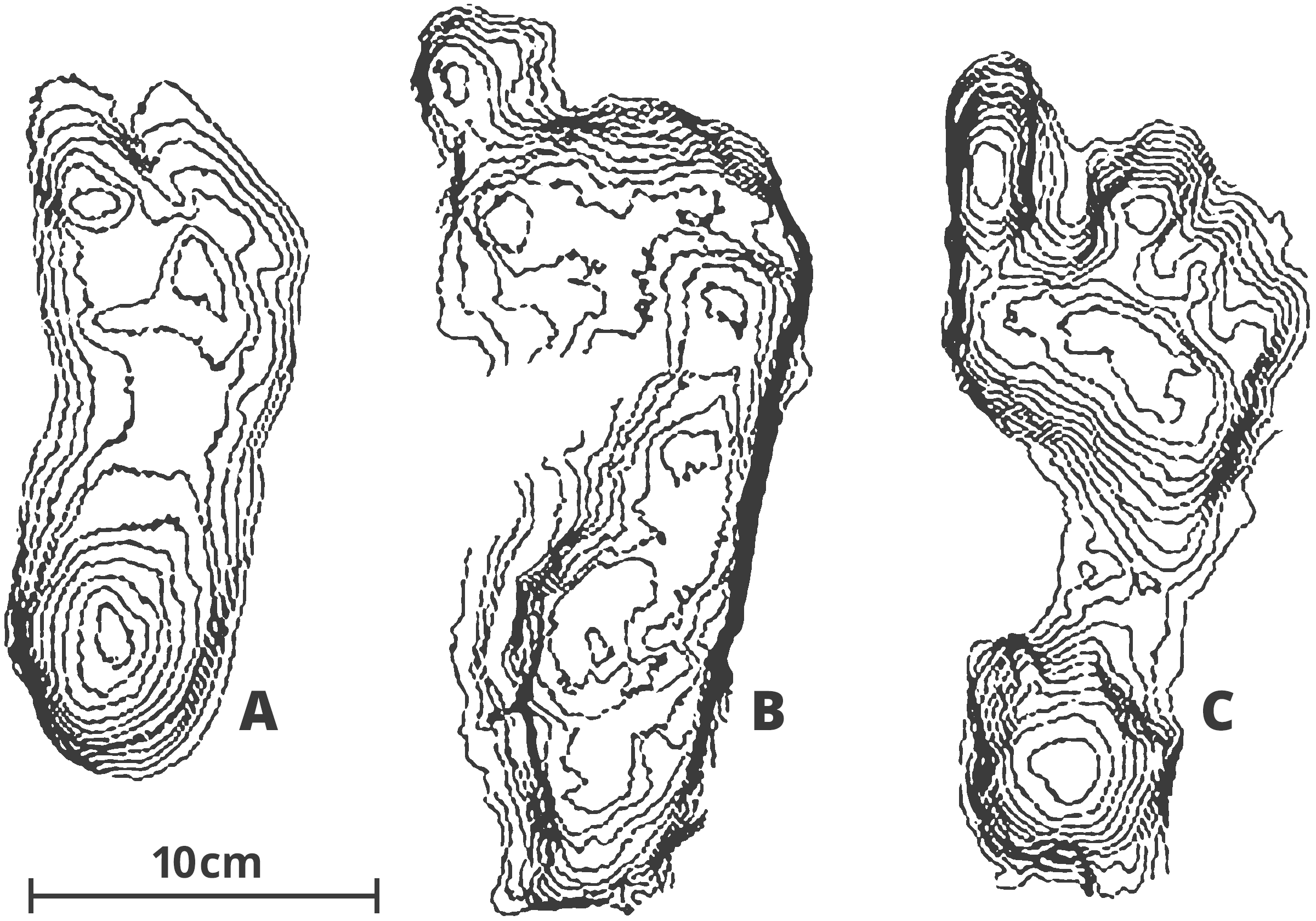
Along with its better preserved and more numerous skeletal material and more clearly bipedal morphology in the pelvis and lower limbs, A. afarensis is the first hominin associated with fossilized trackways. These trackways or footprints date to 3.6 myr, come from a well-preserved layer of volcanic ash in Laetoli, Tanzania (Wood, 2005). Modern human footprints exhibit a derived arched-foot architecture and a stiff-legged striding gait, as illustrated in the fossilized footprints of unshod modern humans in figure 3. Photographs, stereo photographs and casts of the Laetoli footprints demonstrate a mosaic of locomotor characteristics like the more derived reduction of hallucal abduction and the shortening of lateral toes alongside basal chimpanzee-like features such as a flat, flexible midfoot (Meldrum et al., 2011). Together with anatomical evidences, these footprints provided the first direct evidence of bipedality in the human lineage.
The next milestone the human lineage reached after upright walking would be the eventual production and use of tools as well as the associated consumption of meat. The first evidence of meat eating comes from the kerf marks on animal bones found in association with the 2.5 myr hominin
Australopithecus garhi in Ethiopia, cuts that could have only been made using a sharp-edged tool. No stone tools, however, were found in this assemblage, possibly due to the putative low frequency of their production (Wood, 2005). There have been earlier reports from the Afar Valley in Ethiopia of 2.6 myr Oldowan-type stone tools in association with butchered animal bones, but this report has yet to be verified (Semaw et al., 2003). In any event, by the time of
Homo ergaster, reduction of tooth and jaw size indicates that the diet of archaic and transitional humans or the methods used to process food changed. This suggests that
Homo ergaster was perhaps the first human ancestor to routinely cook their food, making naturally tough foods easier to consume. In fact, the earliest evidence of burnt earth in association with stone tools dates to 1-2 myr, roughly the same timeline as
Homo ergaster, although researchers can't rule out the possibility of natural fire being the cause of scorching (Wood, 2005). The importance pinned to tool use, meat-eating and the production of fire is due to the speculation that these features are related to the increase of cognitive capacity and complexity at this stage of human evolution.
In absolute terms, brain size has increased in volume from the last common ancestor, postulated to be a chimp-like average of ~350 cc, to the ~1350 cc of
Homo sapiens. This observation has implications for the development of uniquely human features like culture, language and even the development of motor function, all of which contribute to the phenomenon of modern humanity. As soft tissue, however, the ancient brain cannot be directly studied. Thus, researchers must use indirect methods to study brain evolution and, by extension, the evolution of language. Such approaches include the aforementioned analysis of stone tools, skeletal material such as endocasts, and genetics. Even handedness, or bimanual lateralization, has been implicated as possible evidence for language evolution, as the two traits supposedly share a common anatomical origin (Cashmore, 2009). For the time being, the study of brain evolution is still in its infancy. The advent of modernity, however, can be conceived in other ways.
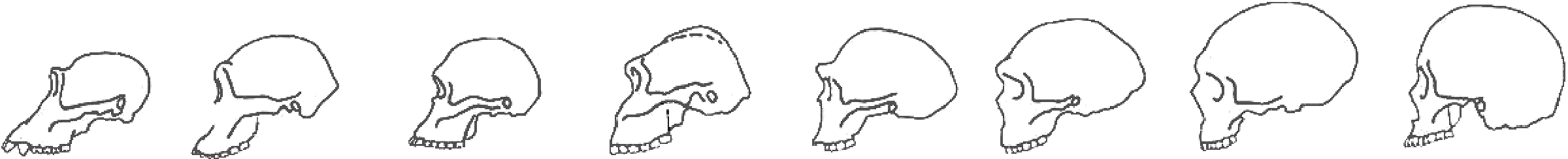
Figure 6: Diagram of Hominin Skulls
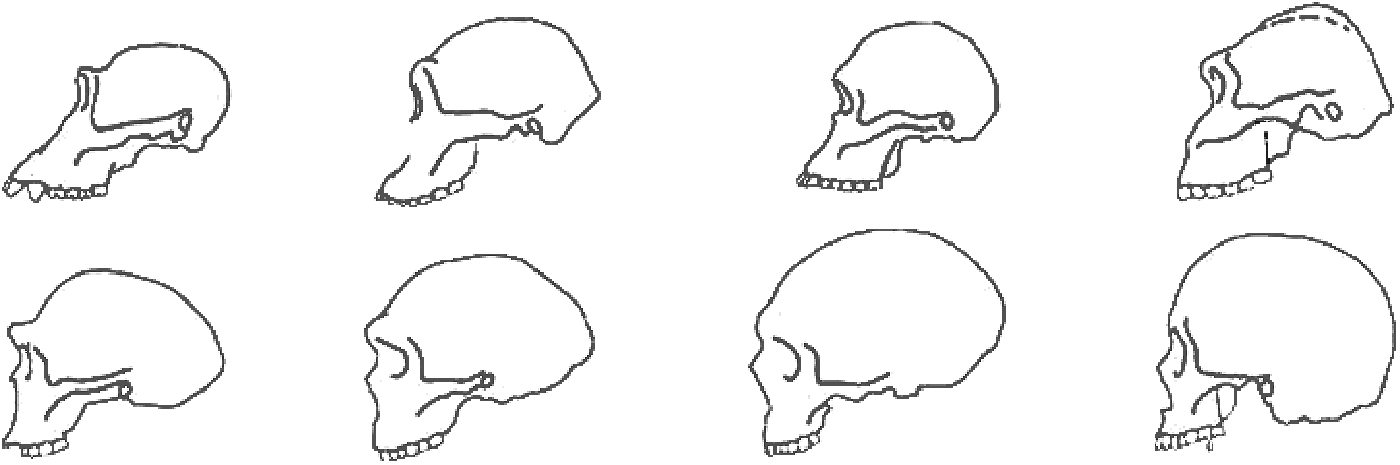
Figure 6: Diagram of Hominin Skulls
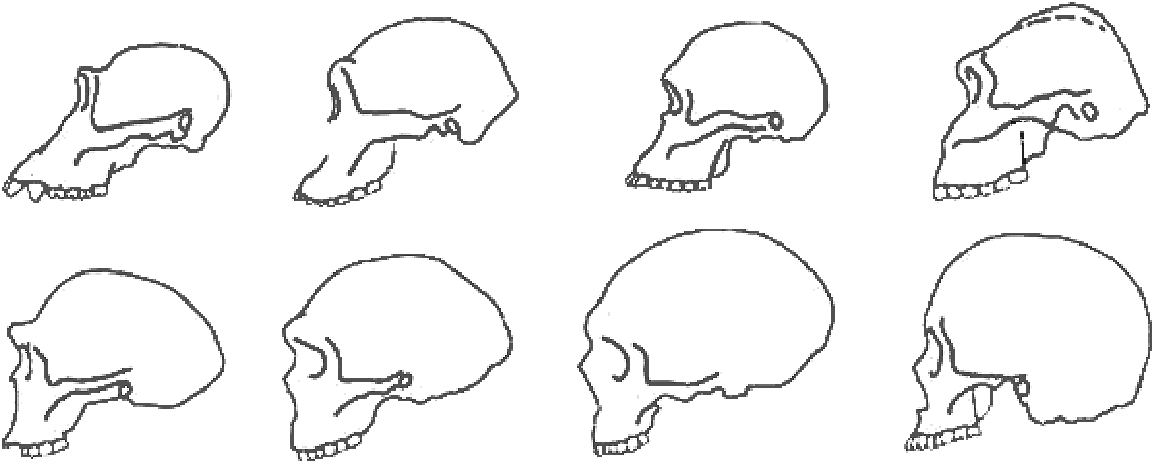
Figure 6: Diagram of Hominin Skulls
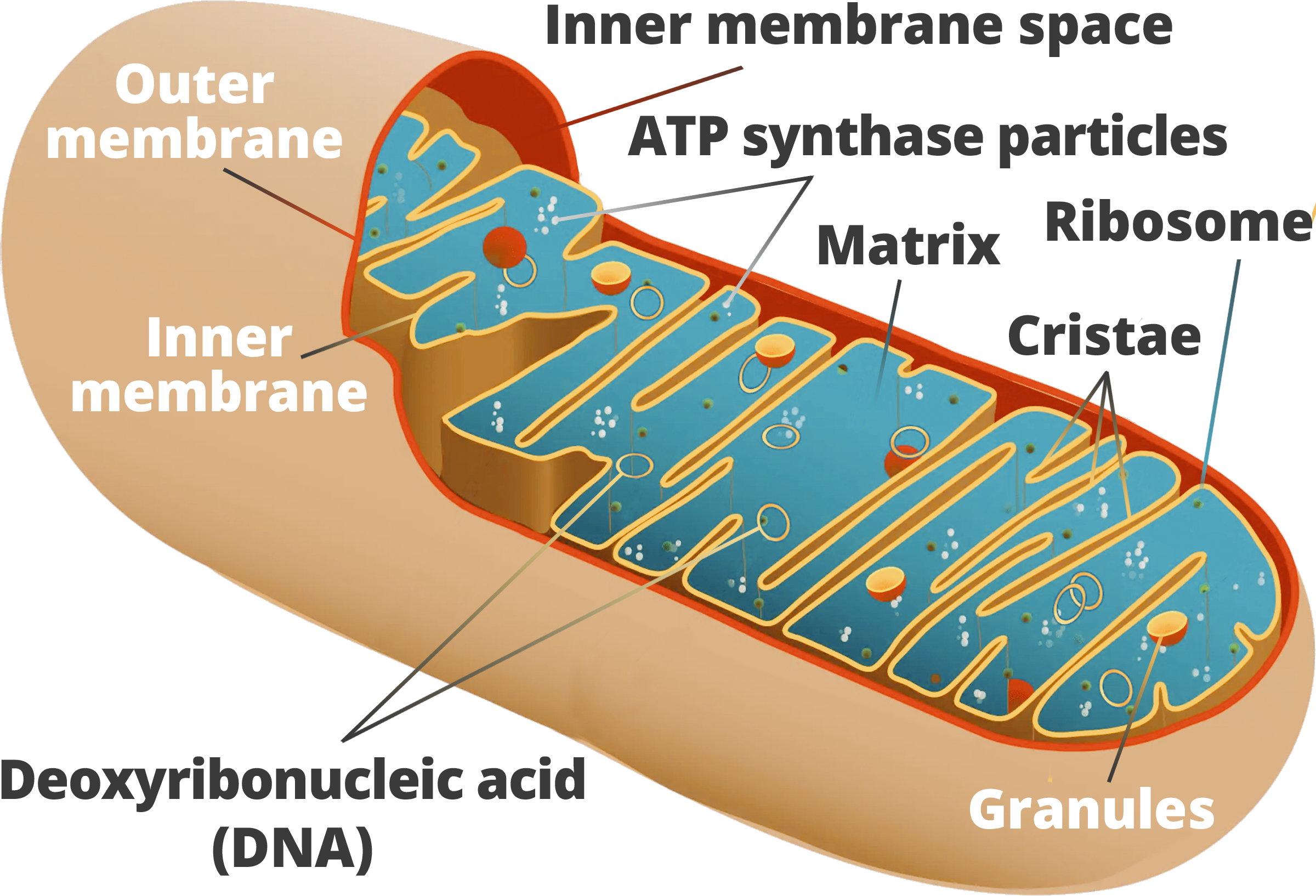
Figure 7: Mitochondrial DNA has demonstrated that the greatest level of genetic diversity in modern humans occurs within the African continent
One way is through dispersal patterns. Africa is considered the homeland of ancestral humans because fossils of this lineage are found there exclusively until the rise of the genus Homo and, more specifically, the colonization of Asian and Indonesian regions by
Homo erectus 1.3-1.8 myr (Wood 2005). Later forms, called
Homo heidelbergensis, managed to penetrate Europe and by 250 kyr had evolved into the form known as
Homo neanderthalensis, or the
Neanderthals. Anatomically
modern humans, however, begin to appear in Africa by about 190 kyr. This species spread out to Asia and Europe only around 50-80 kyr (Jobling, Hurles and Tyler-Smith, 2004). Thus, for approximately 30 thousand years,
Neanderthals and
modern humans existed in the same temporal and geographic space, seemingly rejecting traditional notions that Neanderthals represented the direct ancestor of
modern humans. Two models exist to explain the origin of our genetic modernity, population dispersal and the nature of our relationships with earlier ancestors.

Figure 7: Mitochondrial DNA has demonstrated that the greatest level of genetic diversity in modern humans occurs within the African continent
The Out of Africa model posits that the transition to modern human anatomy occurred in Africa around 200 kyr with what's known as the Mitochrondrial Eve. This is because African genetic diversity is greater than anywhere else in the world and non-African diversity represents just a subset of that which exists in Africa. This is clearly seen in the analysis of mitochondrial DNA lineages, where haplogroups L1 and L2 represent solely African types. Haplogroup L3 is also an African type, but is found outside of the continent as well, along with the descendant haplogroups M and N, which, in addition to their own descendant groups, incorporate all non-African genetic diversity (Gonder et al., 2007; Jobling, Hurles and Tyler-Smith, 2004). Mitochrondrial DNA is unique for several reasons, including the fact that it mutates at a faster rate than nuclear DNA, it doesn't get reshuffled between chromosomes during cell division, it doesn't have the innate mechanisms for DNA repair found in the nucleus and its mutations are largely or entirely under neutral selection. That means that any of the many mutations in mtDNA that have accumulated between two population samples represent a function of how long ago those two populations have been undergoing independent evolution (Wood, 2005; Jobling, Hurles and Tyler-Smith 2004). Researchers have calibrated the divergence point of the L1 haplogroup and the rest to 200 kyr and labeled the first woman with modern human mitochondrial DNA as Mitochondrial Eve.
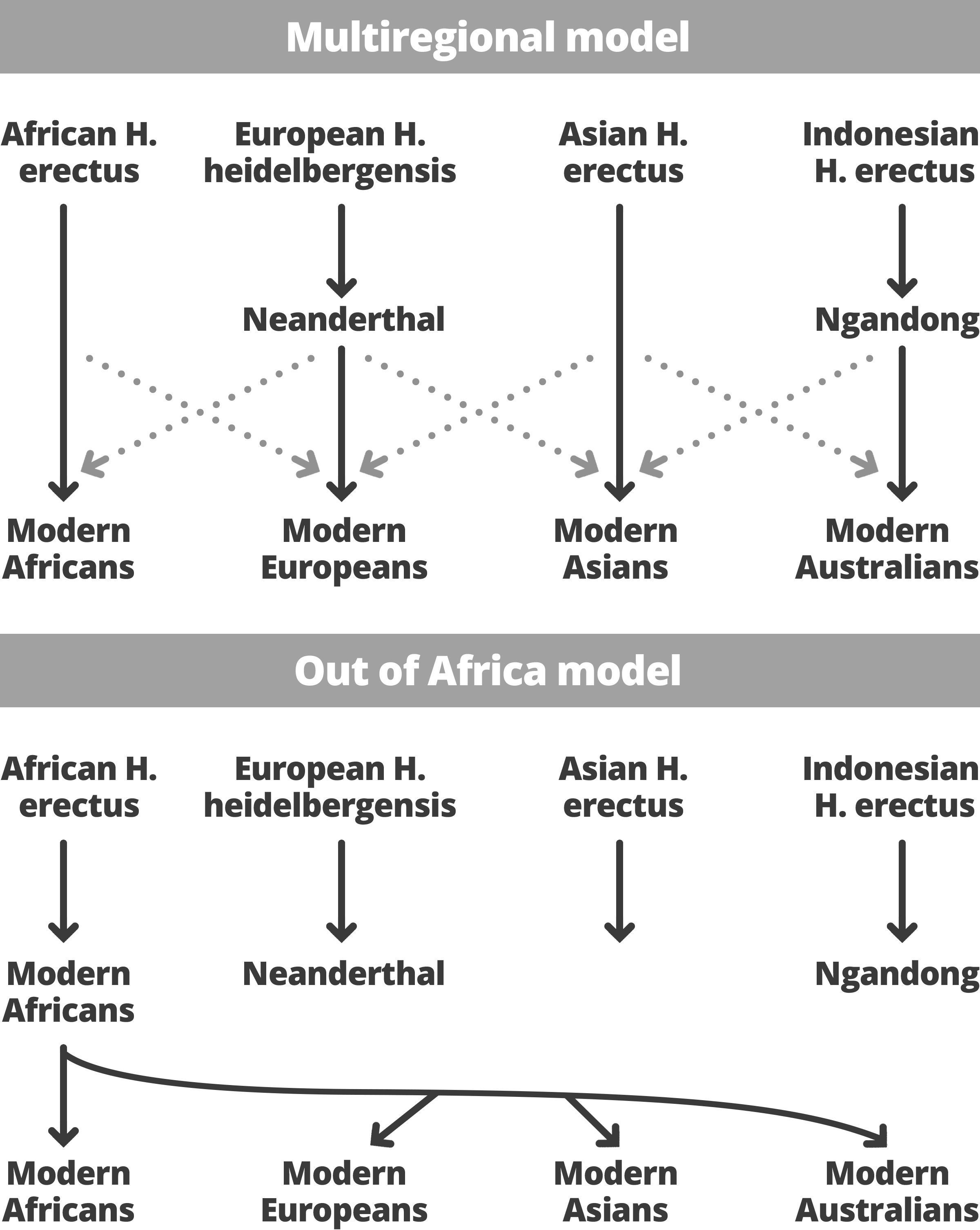
Figure 8: Out of Africa Model - Multiregional Hypothesis
Using Mitochrondrial DNA from various African populations and a global mtDNA reference, Gonder et al. (2007) were able to assign the divergence date of haplogroups L3, M and N to 94.3 +/- 9.9 kyr, roughtly half the amount of time
modern humans have been in existence. This represents another piece of evidence in favor of the Out of Africa model, showing that
modern humans had spent a while in Africa before dispersing into other regions (Gonder et al., 2007). This ties in with the notion that
modern humans replaced the preexisting humans living in Eurasia and Indonesia, such as
Homo erectus, when they left the African continent 100 kyr after the emergence of
Homo sapiens 200 kyr ago (Wood, 2005). Strong versions of the Out of Africa hypothesis also require no genetic admixture, or no mating with the
Homo erectus or
Homo neanderthalensis already in place in the regions
Homo sapiens was exploring (Hurles, Jobling and Tyler-Smith, 2004). Advocates of interbreeding, however, contest problems of contamination, preservation, and the possibility that genetic drift erased
Neanderthal contribution to the modern human genome (Serre, 2004). These principles of replacement and no significant admixture form the basis of the Out of Africa model, contrasting what's known as the Multiregional Hypothesis.
The Multiregional Hypothesis, on the other hand, describes the transition to
modern humans as a process going on across the African, Asian and European continents. This model requires widespread gene flow and posits a genetically interconnected network of human populations across vast distances. Because of the latter point, the model suggests that genetic diversity should be similar in all regions, which the exceptional levels of African mitochondrial diversity effectively falsifies (Wood, 2005). The Multiregional Hypothesis would dictate that each regional variation of ancestral Homo gave rise to the same regional variant of the subsequent species. That is, for example,
Neanderthals of Western Europe would have evolved into
Homo modern humans there but would still be the same species as
modern humans elsewhere due to interbreeding. Analysis of
Neanderthal mitochondrial DNA has shown that it is different from that of
modern humans? with a low level of diversity relative to apes, and that it is no more similar to Europeans than to any other group of
modern humans (Hurles, Jobling and Tyler-Smith, 2004). For that reason, researchers have concluded that the Out of Africa model fits better with the available evidence, although there still exist discrepancies in data that must be resolved before definitive claims are made.
Thus, despite intense interest and research, the details of human evolution remain unclear. Like many paleontological disciplines, definitive conclusions are thwarted by lack of evidence. This is true especially for early hominin evolution, the skeletal evidence for which consists of just a few fragments. The patchy and controversial nature of this record makes it difficult to establish that a fossil can be conclusively included in the hominin lineage. The first unambiguous human ancestor is
Australopithecus afarensis with its bipedal skeletal characteristics and association with the bipedal trackways at Laetoli. Slowly, other distinctly human features arise, ranging from the use of stone tools to encephalization. Even genetic evidence helps to retrace both hominid dispersals and patterns of putative interbreeding typified by the Out of Africa v. Multiregional Hypothesis debate. Of course, gaps in our understanding of human evolution still exist and more data, both physical and genetic, are required before researchers can fill them.
Brunet, Michel; Guy, Franck; Pilbeam, David; Mackaye, Hassane Taisso; Likius, Andossa; Ahounta, Djimdoubalbaye; Beauvilain, Alain; Blondel, Cecile; Bocherens, Herve; Boisserie, Jean-Renaud; de Bonis, Louis; Coppens, Yves; Dejax, Jean; Denys, Christiane; Duringer, Philippe; Eisenmann, Vera; Fanone, Gongdibe; Fronty, Pierre; Geraads, Denis; Lehmann, Thomas; Lihoreau, Fabrice; Louchart, Antoine; Mahamat, Adoum; Merceron, Gildas; Mouchelin, Guy; Otero, Olga; Pelaez Campomanes, Pablo; Ponce de Leon, Marcia; Rage, Jean-Claude; Sapanet, Michel; Schuster, Mathieu; Sudre, Jean Tassy, Pascal; Valentin, Xavier; Vignaud, Patrick; Viriot, Laurent; Zazzo, Antoine and Christoph Zollikofer (2002) A New Hominid from the Upper Miocene of Chad. Nature 418: 145-151.
Cashmore, Laura (2009) Can Hominin 'Handedness' be accurately assessed? Annals of Human Biology 36: 624-641.
Cobb, Samuel N (2008) The Facial Skeleton of the Chimpanzee-Human Last Common Ancestor. Journal of Anatomy 212: 469-485.
Gonder, Mary Katherine; Mortensen, Holly M; Reed, Floyd A; de Sousa Alexandra and Sarah Tishkoff (2007) Whole-mtDNA Genome Sequence Analysis of Ancient African Lineages. Molecular Biology and Evolution 24.3: 757-768.
Harcourt-Smith, William HE (2010) The First Hominins and the Origins of Bipedalism. Evolutionary Education Outreach 3: 333-340. Kappelman, John (1996) The Evolution of Body Mass and Relative Brain Size in Fossil Hominids. Journal of Human Evolution 30: 243-276.
Lovejoy, C. Owen; Simpson, Scott W.; White, Tim D.; Asfaw, Berhane and Gen Suwa (2009) Careful Climbing in the Miocene: The forelimbs of Ardipithecus ramidus and humans are primitive. Science 326: 70-70e8.
Lovejoy, C. Owen; Suwa, Gen; Simpson, Scott W.; Matternes, Jay H. and Tim D. White (2009b) The Great Divides: Ardipithecus ramidus reveals the postcrania of our last common ancestor with African apes. Science 326: 73, 100-106.
Lovejoy, C. Owen; Suwa, Gen; Spurlock, Linda; Asfaw, Berhane and Tim D. White (2009c) The Pelvis and Femur of Ardipithecus ramidus: The emergence of upright walking. Science 326: 71-71e6.
Meldrum, DJ; Lockley, Martin G; Lucas, Spencer G and Charles Musiba (2011) Ichnotaxonomy of the Laetoli Trackways: The earliest hominin footprints. Journal of African Earth Sciences 60: 1-12.
Semaw, Sileshi; Rogers, Michael J; Quade, Jay; Renne, Paul R; Butler, Robert F; Dominguez- Rodrigo, Manuel; Stout, Dietrich; Hart, William S; Pickering, Travis and Scott W Simpson (2003) 2.6 Million Year Old Stone Tools and Associated Bones from OGS-6 and OGS-7, Gona, Afar, Ethiopia. Journal of Human Evolution 45.2: 169-177.
Senut, Brigitte; Pickford, Martin; Gommery, Dominique; Mein, Pierre; Kiptalam, Cheboi and Yves Coppens (2001) Premier hominidé du Miocène (formation de Lukeino, Kenya). Comptes Rendus de l'Académie des Sciences 332.2: 137-144.
Serre, David; Langaney, Andre; Chech, Mario; Teschler-Nicola, Maria; Paunovic, Maja; Mennecier, Philippe; Hofreiter, Michael; Possnert, Goran and Svante Paabo (2004) No Evidence of Neandertal mtDNA Contribution to Early Modern Humans. PLOS Biology 2.3: 313-318.
Steiper, Michael E and Nathan M Young (2006) Primate Molecular Divergence Dates. Primate Molecular Phylogenetics and Evolution 41: 384-394.
Wood, Bernard (2005) Human Evolution: A very short introduction. Oxford University Press, Oxford.